Florida ECT Device Manufacturer Flouted FDA Regulations, FDA Inspection Documents Reveal
Somatics LLC comes under scrutiny amid heightened controversy over ECT (electroconvulsive treatment).
Thanks to documents just released from a recent Freedom of Information Act request, Food and Drug Administration (FDA) inspection reports are now available showing Somatics LLC failed to comply with the agency’s orders and regulations.
ECT device manufacturer, Somatic, ignored mandatory procedures to report instances of the devices harming patients. It also exceeded marketing regulations and downplayed severe risks such as permanent memory loss, in its promotional literature.
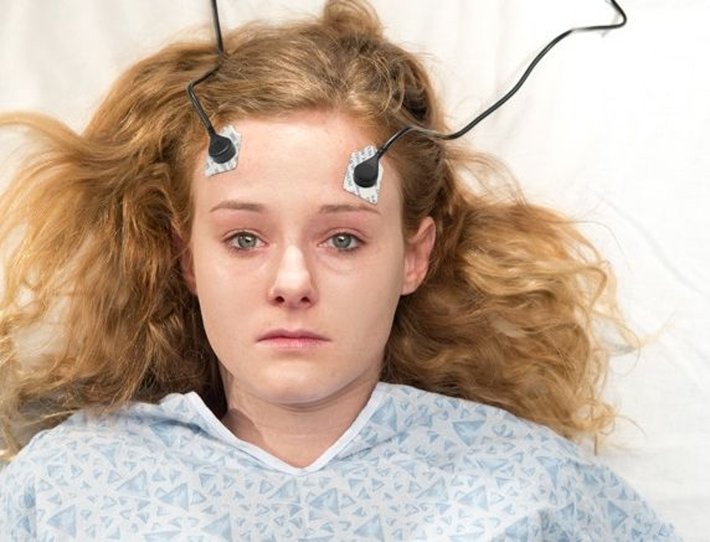
A highly controversial procedure, ECT involves shooting up to 450 volts of electricity through the brain—33 times the amount employed in the kind of torture administered to Abu Ghraib prisoners.
The Freedom of Information Act request was submitted on behalf of Citizens Commission on Human Rights, a mental health watchdog founded in 1969 by the Church of Scientology and Dr. Thomas Szasz, a renowned professor of psychiatry.
A highly controversial procedure, ECT involves shooting up to 450 volts of electricity through the brain—33 times the amount employed in the kind of torture administered to Abu Ghraib prisoners. According to the FDA, ECT can cause cognitive impairment, memory impairment, prolonged seizures, dental trauma, manic symptoms, pulmonary complications, worsening of psychiatric symptoms, and death. One third of ECT patients experience permanent memory loss and many suffer a steep drop in IQ.
The procedure was described as torture “in the guise of rehabilitation” in a 2013 report, published by the United Nations High Commissioner for Human Rights Committee Against Torture.
The first inspection performed by the FDA at Somatics took place in 2012, 28 years after the firm began marketing its ECT device. The inspection uncovered 11 regulatory violations.
One of the more egregious violations was a lack of a required system to allow patients to report complaints. The report states that Somatics LLC “does not include an internal system which provides for the timely and effective identification, communication, and evaluation of events that may be subject to medical device reporting requirements” and “complaint records do not include required information.”
According to the FDA website, Medical Device Reporting (MDR) is a tool the FDA “uses to monitor device performance, detect potential device-related safety issues, and contribute to benefit-risk assessments” of medical devices. Manufacturers of devices “are required to submit certain types of reports for adverse events and product problems to the FDA.” No Medical Device Reports have ever been filed by Somatics to the FDA’s “MAUDE” database (Manufacturer and User Facility Device Experience Database).
The FDA agent who carried out the 2012 inspection warned Somatics “that it was the firm’s responsibility to investigate all complaints,” which was not being done.
Documents further show that Somatics LLC’s device order form includes Dr. Richard Abrams’ Electroconvulsive Therapy which claims to be “a clinical guide to ECT.” The book advises those using the device to “never allow themselves to be bound solely by the narrow confines of data from controlled trials.” The book also recommends employing ECT for uses not approved by the FDA and also claims: “No long-term or persistent effects of ECT on intellectual abilities or memory capacity have been shown to occur.”
Yet Freedom Magazine cites a survey of ECT practitioners by the American Psychiatric Association (APA), revealing that as many as 41 percent of respondents agreed that “electroshock caused brain damage.”
In April 1982, the FDA gave ECT device manufacturers 30 months to hold standard clinical trials to prove the device was safe and effective. Somatics and other ECT device manufacturers never complied with this order. Nevertheless, in December 2015, the FDA issued a proposed order to “down-classify” the device used to deliver electroshock therapy, a move followed by a wave of public outcry.
On behalf of five individuals who were damaged by electroshock, constitutional attorney Jonathan Emord has filed a Citizens Petition with the FDA to prevent the reclassification.
According to the petition, in 2009, when the FDA opened a public docket for information and comments on the reclassification of ECT devices:
- The FDA received more than 3,000 comments, most of which (79%) opposed reclassifying ECT devices to Class II.
- The comments related hundreds of stories of permanent memory loss, deaths of loved ones and the debilitating effects of ECT.
- There were 92 group submissions representing 6,462 individuals against reclassification and 462 in favor.
- The adverse reactions reported were memory adverse event (529), other cognitive complaint (413), brain damage (298) and deaths (103).
A feature-length exposé on electroconvulsive therapy, ECT device manufacturers and FDA corruption by award-winning journalist Ajay Singh appears in the October–November 2016 edition of Freedom Magazine.
Citizens Commission on Human Rights is dedicated to eradicating abuses committed under the guise of mental health and enacting patient and consumer protections.
The Scientology religion was founded by author and philosopher L. Ron Hubbard. The first Church of Scientology was formed in Los Angeles in 1954 and the religion has expanded to more than 11,000 Churches, Missions and affiliated groups, with millions of members in 167 countries.
CONTACT:
Church of Scientology Media Relations
mediarelations@churchofscientology.net
(323) 960-3500 phone
(323) 960-3508 fax

